ABSTRACT
Although idiopathic pulmonary fibrosis remains a devastating diagnosis, recent advances have improved our understanding of many facets of this disease. These breakthroughs, combined with the increased general availability of therapeutic trials, hold the promise of a brighter future for idiopathic pulmonary fibrosis patients. For example, we now have a more comprehensive understanding of the diagnostic criteria and natural history of the disease. Several studies have shown that simple measurement of pulmonary physiology or gas exchange can be used to predict patient survival. By identifying several molecular pathways that play significant roles in the pathogenesis of idiopathic pulmonary fibrosis, investigators have produced a growing list of novel potential therapeutic targets for the disease. Several prospective, controlled therapeutic trials have been conducted. Others are ongoing or are still in the planning stages. These efforts have advanced our current knowledge of idiopathic pulmonary fibrosis and have raised new important questions, as well as having generated the interest and momentum needed to gain additional ground in the fight against this challenging disease. This article offers the reader a view of the recent advances in idiopathic pulmonary fibrosis research, with a focus on natural history, pathogenesis and treatment.
Keywords:
Pulmonary fibrosis/diagnosis; Pulmonary fibrosis/drug therapy; Fibroblasts; Transforming growth factor beta; Lung/pathology, Anti-inflammatory agents/therapeutic use
RESUMO
Embora diagnósticos de fibrose pulmonar idiopática continuem sendo devastadores, avanços recentes têm melhorado nossa compreensão a respeito de muitas das facetas desta doença. Estas descobertas, juntamente com o aumento da disponibilidade geral de ensaios terapêuticos, encerram a promessa de um futuro mais promissor para pacientes com fibrose pulmonar idiopática. Por exemplo, nós temos agora uma compreensão mais abrangente a respeito dos critérios diagnósticos e da história natural da doença. Vários estudos têm mostrado que a mensuração simples da fisiologia pulmonar ou troca gasosa pode ser usada para prever a sobrevida do paciente. Através da identificação de várias vias moleculares que têm papéis importantes na patogênese da fibrose pulmonar idiopática, os pesquisadores têm produzido uma lista crescente de possíveis novos alvos terapêuticos para a doença. Vários ensaios terapêuticos prospectivos e controlados têm sido realizados. Outros estão em andamento ou ainda estão em fase de planejamento. Estes esforços têm avançado nosso conhecimento atual sobre fibrose pulmonar idiopática e levantado novas questões importantes, assim como têm gerado o interesse e o impulso necessários para avançar terreno na luta contra esta doença desafiadora. Este artigo oferece ao leitor um panorama dos avanços recentes nas pesquisas sobre fibrose pulmonar idiopática, tendo como foco a história natural, patogênese e tratamento.
Palavras-chave:
Fibrose pulmonar/diagnóstico; Fibrose pulmonar/farmacoterapia; Fibroblastos; TGF-b: Pulmão/patologia; Agentes anti-inflamatórios/uso terapêutico
INTRODUCTIONIdiopathic pulmonary fibrosis (IPF) is the most common of the idiopathic interstitial pneumonias and, unfortunately, carries the worst prognosis.(1) Its estimated prevalence, around 30 cases per 100,000 people, increases precipitously with age and affects more than 100 individuals per 100,000 people aged 75 years or more.(2) Hallmark symptoms include a nagging, dry cough and progressive breathlessness. A median survival of about three years from the time of diagnosis can be expected. (3)
In the past few years, several important clinical and pathobiologic discoveries have been made about IPF. These findings have advanced our understanding of the diagnosis, natural history, and molecular underpinnings and produced an inventory of potential therapeutic targets needing further study. Also, recent research has helped generate increased public awareness of IPF, creating an environment where clinical trials are now more commonplace. These efforts have provided the impetus for the development of a United States National Institutes of Health (NIH)-funded IPF clinical research network (IPFnet). This consortium of institutions focused on IPF research is charged with conducting large trials of individual and combination therapies, in the hope of identifying an effective treatment for this devastating disease.
The objective of this review is to highlight some of the recently completed and ongoing research in IPF.
DIAGNOSIS AND NATURAL HISTORYDiagnosis
In 1999, members from the American Thoracic Society (ATS) and European Respiratory Society (ERS) collaborated to review over 3500 articles published in the scientific literature for information pertaining to IPF's physiology, radiology, and pathology; its pathogenesis, epidemiology, clinical presentation, and staging; its inheritance or familial occurrence; its treatment; and its prognosis.(4) These experts produced an international consensus statement that comprehensively discusses IPF and includes criteria that can be used to make a diagnosis of IPF with and without performing a lung biopsy (Chart 1).

While, in the correct clinical setting, a confident diagnosis of IPF can be made without a surgical lung biopsy, it remains necessary in patients less than 50 years old or in those without a characteristic disease pattern (Figure 1) on chest high resolution computed tomography (HRCT).(5)
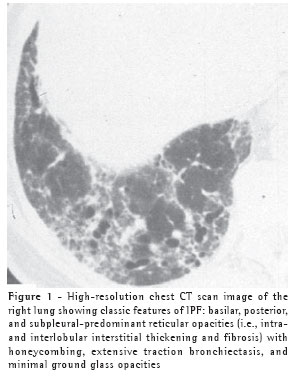
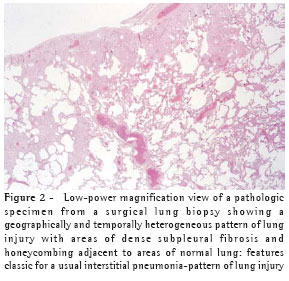
When a biopsy is performed, the usual interstitial pneumonia (UIP) histopathologic pattern (Figure 2) in at least one specimen is necessary to make a definitive diagnosis of IPF. Care must be exercised to rule out alternative clinical explanations for this UIP-pattern as it is not unique to IPF (Chart 2).

Prognosis
Baseline clinical variables. Several baseline (at the time of diagnosis) clinical variables are known to predict survival in patients with IPF. These include age, symptom severity, disease duration, radiographic features, and pulmonary physiology. New data have shown that gas exchange as measured by desaturation and its severity(6) during a timed-walk test provide important prognostic information.
In a study of 83 patients, including 22 with IPF, Lama et al., revealed the prognostic value of desaturation during a 6MWT.(6) Among subjects with IPF whose peripheral oxygen saturation (SpO2) fell to 88% or less ("desaturators") during the 6MWT on room air, the four-year survival rate was 34.5%. This contrasts with a four-year survival rate was 69.1% in those who did not desaturate (Figure 3). IPF desaturators experienced a greater than four-fold hazard of death compared with "non-desaturators." When desaturation status was included in a multivariable model, age, gender, history of smoking, diffusing capacity for carbon monoxide (DlCO), forced vital capacity (FVC), and resting saturation were not independent predictors of survival. The severity of desaturation also appears to predict survival.(7)
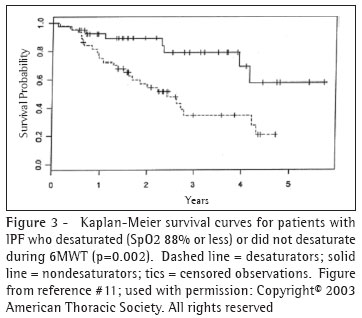
These findings are somewhat tempered by the findings of Eaton and colleagues who recently showed that lowest SpO2 during a 6-minute walk test was not particularly reliable (r=0.61) at one week.(8) In contrast, they found that distance walked during six-minute walk test had excellent reproducibility at one week (r=0.98). The distance walked correlated significantly with maximal oxygen uptake measured during symptom-limited treadmill exercise test as well as DICO but not with SpO2.
Dynamic clinical variables. Changes in clinical variables over time have shown to be useful predictors of outcome (Chart 3). It has been known for a number of years that a response to therapy over an initial three- to six-month observation period (as measured by symptoms, radiology, and physiology) is associated with prognosis.(9) Now, further investigation has shown that changes in dyspnea or easily measured pulmonary physiology perform well-significantly better than baseline values-in predicting survival.
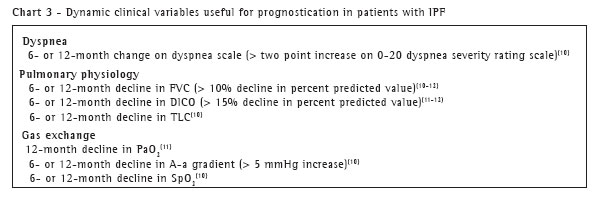
In a retrospective cohort study of IPF patients evaluated at the National Jewish Medical and Research Center in Denver, Colorado, investigators found that six- and twelve-month changes from baseline in dyspnea score, total lung capacity (TLC), FVC, partial pressure of arterial oxygen, arterial oxygen saturation, and alveolar-arterial oxygen gradient [(A-a )O2)] independently predicted survival when controlling for baseline values.(10)
The most powerful predictors of survival in this study were six-month changes from baseline in dyspnea score, percent predicted FVC (ppFVC), and (A-a) O2. For example, in 30 IPF patients whose six-month ppFVC declined by a percent predicted value of 10 or more, only 22% were alive at five years, compared with 68% of patients whose ppFVC increased by ten or more.
In a similar, well-defined cohort of 63 IPF patients and 41 patients with idiopathic nonspecific interstitial pneumonia (NSIP) from the Royal Brompton Hospital in London, 12-month changes in percent predicted diffusing capacity for carbon monoxide (ppDLCO) and ppFVC were stronger predictors of mortality than other variables, including age, gender, and histopathological pattern.(11) Flaherty and co-workers analyzed data from 80 patients with IPF and 29 patients with idiopathic NSIP and found that a change in the absolute value (rather than percent predicted) of FVC, from the time of lung biopsy to six months post-biopsy, was a strong predictor of survival.(12) Regardless of pathology, patients whose FVC decreased from baseline by 10% or more at six-months had a hazard ratio for death two and one-half times that for patients whose FVC either remained stable or increased at six months.
Pulmonary hypertension. Another topic that is gaining attention is secondary pulmonary arterial hypertension (PAH). Investigators are eager to determine how PAH effects prognosis in patients with IPF.(13) If an association with a worse prognosis is confirmed, it will be interesting to observe whether the medications which have improved survival in patients with primary pulmonary hypertension (e.g. inhaled prostanoids, sildenafil), will have the same beneficial effects on patients with PAH associated with IPF. In one randomized, controlled, open-label study, investigators compared the pulmonary vasodilatory effects of a single 50mg dose of sildenafil with escalating doses of intravenous epoprostenol in 16 patients with IPF and lung fibrosis-induced PAH.(14) Compared to epoprostenol, sildenafil produced a more favorable hemodynamic profile; although both drugs decreased pulmonary vascular resistance, sildenafil decreased the ratio of pulmonary to systemic vascular resistance, while that ratio remained unchanged in patients receiving epoprostenol. In addition, sildenafil maintained ventilation perfusion matching, whereas epoprostenol induced ventilation perfusion mismatching. Other trials of potentially efficacious agents aimed at IPF-induced PAH are ongoing (and some are discussed in sections to follow), but until data become available, the important questions about PAH in IPF remain unanswered.
Acute exacerbations. In the past, the natural history of IPF in the vast majority of patient was assumed to be one of gradual and inexorable progression of disease. However, for years clinicians have recognized that the clinical course can vary widely from patient to patient. In contrast to a slow and progressive decline, it is now appreciated that in at least some patients, worsening occurs in a stair step pattern, in which lung function remains stable ("plateaus") between episodes of more abrupt decline.
These abrupt declines in function or "acute exacerbations of IPF" can be of varying severity, occur randomly, and are independent of physiologic stage.(15) These episodes are defined by the acute or subacute onset (i.e., within four weeks) of three factors: 1) progressive dyspnea, 2) new, diffuse radiographic opacities, and 3) worsening gas exchange in the absence of infection or other known causes.(16-17) Surgical lung biopsy in cases of acute exacerbations show a pattern of acute lung injury (diffuse alveolar damage)-with or without hyaline membranes-on a background of a UIP pattern.(17) The UIP-pattern may or may not be entirely obscured by the acute injury.
Martinez et al.,(15) recently examined the placebo group (n=168) from a multi-center, randomized, controlled trial of interferon-gamma (IFN-g) 1b for IPF. On retrospective analysis, of the 36 deaths in this placebo group, 89% were considered to be IPF-related, and nearly half of these IPF-related deaths were due to acute exacerbations of disease.(15)
PATHOGENESISMounting evidence suggests that, in IPF, an inflammatory response neither precedes nor plays an instrumental role in the progression of lung fibrosis.(18-19) The current paradigm hypothesizes that IPF is an "epithelial-fibroblastic" disease.(18) In this model, complex interactions between, alveolar epithelial cell injury and mesenchymal cells result in dysregulated repair mechanisms, with excess pro-fibrotic cytokines, the overproduction of extracellular matrix, and disordered angiogenesis.
Alveolar epithelial cells. Although the fibroblast is recognized as a key cell in the pathogenesis of IPF, there has been renewed interest in the role of the alveolar epithelial cell. In IPF, epithelial cells are injured and in response, release several cytokines. These cytokines result in multiple effects, including activation and proliferation of fibroblasts.(19) Beyond their effects on neighboring cells, the phenomenon of epithelial-mesenchymal transformation (EMT) also appears to occur.(20) In EMT, alveolar epithelial cells transdifferentiate into fibroblasts and myofibroblast-like phenotypes. A clearer understanding of the role of EMT in the pathogenesis of IPF will be important for identifying more specific therapeutic targets.
Fibrocytes. Multiple stimuli are capable of driving the differentiation of fibroblasts into myofibroblasts-alpha-smooth muscle actin producing cells and that appear to be the drivers of exuberant collagen production and deposition seen in IPF. It was previously thought that the fibroblasts and myofibroblasts in IPF were resident to the lung; however, exciting new data suggest that circulating, bone marrow-derived cells-originally discovered and named "fibrocytes" by Bucala et al., in 1994(21) traffic to the lungs in response to injury and also participate in the evolution of fibrosis in IPF.(22-23) These data are consistent with the lung repair role of circulating stem cells proposed by Suratt et al.(24) The precise role of circulating fibrocytes in relation to resident lung fibroblasts in the pathogenesis of IPF has yet to be fully elucidated.
Fibroblast reticulum. In the UIP-pattern of lung injury, subepithelial aggregations of fibroblasts and myofibroblasts, called fibroblast foci, are believed to represent the leading edge of fibroproliferation.(25) Proposed by Myers and Katzenstein(26) to be important in the development and progression of disease, the numbers of these foci seen on surgical lung biopsy have proven to be associated with both physiologic progression of disease(27) and prognosis.(28-29) On two dimensional histologic section, these foci appear isolated and randomly distributed throughout the interstitium. However, recent evidence presented at the 2004 International Meeting of the American Thoracic Society, suggests that, when the UIP lung is reconstructed in three dimensions, individual foci are actually a complex, interconnected reticulum. More investigation is necessary to clarify how or whether there is molecular cross-talk between the elements composing this fibrotic framework.
TAKING AIM: NEW THERAPEUTIC TARGETSRationale for a targeted approach to therapy for IPF
Mounting evidence suggests that the cellular inflammatory response plays only a minimal role in the pathogenesis of IPF: inflammation is not required to produce fibrosis in the lungs in vitro;(30-31) inflammation is sparse in lung pathologic specimens with UIP-patterns of lung injury; and decades of treating IPF patients with anti-inflammatory medications has not resulted in improved survival for patients.(32) Given our current understanding, a more targeted approach is warranted.
Ideally, a biologic target in IPF should fulfill three criteria: 1) there should be strong biologic evidence supporting the target as important in pathogenesis; 2) it should be differentially expressed in the presence and absence of disease; and 3) altering the level (or activity) of the target should lead to modification of disease development or its course.(33) As we currently understand IPF, the beneficial manipulation of an appropriate target should result in limiting or even reversing the amount of lung fibrosis. Several potential mechanisms are considered appropriate sites for investigation and intervention, including: 1) decreasing lung fibroblast migration, proliferation, and differentiation; 2) decreasing the synthesis and deposition of extracellular matrix (ECM); 3) increasing the degradation of aberrantly deposited ECM; 4) enhancing fibroblast/myofibroblast apoptosis; 5) inhibiting inappropriate alveolar epithelial cell apoptosis; 6) enhancing alveolar basement membrane re-epithelialization; 7) disrupting EMT; and 8) modulating angiogenic activity. Within these pathways, particular molecular targets are attractive candidates for modulation and include: transforming growth factor-beta (TGF-b), glutathione, tumor necrosis factor-alpha (TNF-a), endothelin, and several others (Chart 4).(34)
Recent therapeutic trials
There is a strong, and growing commitment in the United States to conduct clinical trials via rigorously developed collaborations. The National Heart Lung and Blood Institute of the National Institutes of Health recently committed over $30 million to support an IPF clinical research network (IPFnet). The network will consist of several "clinical centers" and one "data and coordinating center." It is hoped that this network will offer the opportunity for hundreds of newly diagnosed IPF patients per year to enroll in controlled trials. While IPFnet will assist in the further investigation of therapeutic alternatives in IPF, there have been a large number of recently completed, or soon-to-be-completed trials that inform our current understanding of treatment options.
Interferon-beta 1a. In the United States, interferon-beta 1a (IFN-b) is approved for the treatment of multiple sclerosis. To examine its potential beneficial therapeutic effects in IPF, investigators conducted a multi-centered, randomized, double blind, placebo-controlled, dose-ranging study of 167 IPF patients from 34 sites in the United States and Canada.(35) While negative- IFN-b failed to show a significant benefit in pulmonary function, arterial oxygenation, disease progression, or dyspnea-this trial was the first to demonstrate that llarge, well-designed, multi-centered placebo-controlled trials in IPF are feasible.
Interferon-gamma 1b. Interferon-gamma 1b (IFN-g) is a cytokine produced by T lymphocytes and natural killer cells.(36) It has several anti-fibrotic properties, such as an inhibitory effect on fibroblast proliferation and activation,(37-38) including that which is induced by TGF-b.(39) Early clinical evidence suggesting that IFN-g might be a useful therapy for patients with IPF appeared in a small, randomized, prospective trial in which 18 IPF patients received prednisone 7.5mg daily and either 200mcg of IFN-g subcutaneously three times weekly or matching placebo.(40) The results revealed significant and clinically meaningful improvements in pulmonary physiology and gas exchange in the treatment group. These promising results led to a large-scale, multi-centered, randomized, double-blinded, placebo-controlled trial, in which 330 patients were randomized to either IFN-g or placebo.(41) IFN-g had no effect on the primary combined endpoint of either a decrease in FVC greater than 10%, an increase in resting ambient air (A-a) O2 gradient, or death. In post-hoc analyses, when survival was examined as a lone endpoint, there appeared to be a trend (i.e., log rank p=0.08) toward increased survival in the actively treated group (Figure 4). Additionally, of the 254 patients with a FVC greater than 55% of predicted normal, 21 of 128 patients (16.4%) in the placebo group died, while only 6 of 126 subjects (4.8%) in the IFN-g group died (p=0.004).
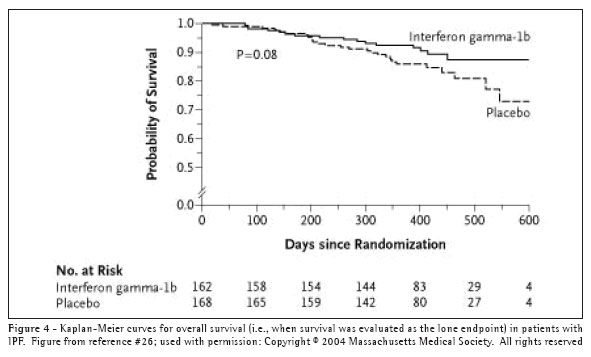
The encouraging results of these post hoc analyses have led to a larger (800 patient), phase III trial of IFN-g with mortality as the primary endpoint.
Pirfenidone. Pirfenidone is an investigational, small pyridine molecule with anti-inflammatory and anti-fibrotic effects. Although pirfenidone's exact mechanism of action is unknown, its anti-fibrotic effects include inhibiting TGF-b-stimulated collagen synthesis, decreasing extracellular matrix production, and blocking the mitogenic effects of pro-fibrotic cytokines on fibroblasts.(42) In an initial phase II, single-center, open-label study of 54 patients who had refused, not tolerated, or failed to respond to conventional immunosuppressive therapy, pirfenidone appeared to have favorable effects on lung function.(43) Twenty-nine of 41 patients for whom data were available had stable (n=19) or improved ppFVC (10% or greater increase) at six months. Adverse effects of pirfenidone were relatively minor; the most common being nausea and photosensitivity dermatitis. These promising results provided impetus for examining pirfenidone in larger trials.
The results of a subsequent placebo-controlled, randomized, 25-site trial of pirfenidone in Japan, provide further encouragement.(44) One hundred seven patients with well-defined IPF were randomized to pirfenidone or placebo. Patients could also take prednisone in doses of 10mg/day or less, but they could not take other anti-inflammatory or anti-fibrotic drugs.
The trial was stopped by the investigators at the recommendation of the data safety monitoring board when it was discovered that five patients in the placebo group, but no patients in the pirfenidone group, had acute exacerbations of IPF. At nine months, there was a trend toward statistical significance in the primary endpoint-change in the lowest SpO2 during a 6MWT-favoring pirfenidone. The SpO2 in the group receiving pirfenidone increased by 0.47 +/- 3.9%, while the SpO2 in the group receiving placebo decreased by 0.94 +/- 3.4% (p=0.07). From baseline to nine months, mean values for vital capacity (VC), TLC, and DLCO declined in both groups; VC declined significantly less in the pirfenidone group (30cc decline versus 130cc in the placebo group, p=0.036). There were no differences between groups in dyspnea scores or quality of life. In a pre-specified subset of all patients who were able to complete the 6MWT without desaturation below 80% (n=80, 55 in pirfenidone group and 25 in the placebo group), the difference between groups in lowest SpO2 during 6MWT reached statistical significance at nine months (p=0.03). These results suggest that pirfenidone warrants further systematic investigation in a carefully developed longitudinal trial.
N-acetylcysteine (NAC). Evidence suggests there is an imbalance between oxidation products and antioxidants in the lungs of patients with IPF.(45) Glutathione is an antioxidant, which, in reduced form, mitigates oxidant-induced cellular injury, and glutathione levels are decreased in the epithelial lining fluid of patients with IPF.(46) Behr et al., showed that NAC, a metabolic precursor of glutathione, could increase glutathione levels in bronchoalveolar lavage and epithelial lining fluid from the lungs of patients with pulmonary fibrosis.(45) In one study, ten patients with IPF and ten with connective tissue disease-associated pulmonary fibrosis (CTD-fibrosis), had oral NAC (600mg three times daily) added to their conventional immunosuppressive drug regimens.(46) After 12 weeks of NAC, ppDLCO improved significantly (from 56.5 +/- 4.4% at baseline to 61.4 +/- 4.6%, p<0.05), but other measures of pulmonary physiology and gas exchange remained unchanged.
In follow-up to this early trial, a prospective, randomized, placebo-controlled, multi-centered trial compared the effect on FVC and DlCO of 12 months of a regimen containing prednisone, azathioprine, and NAC versus a regimen of prednisone, azathioprine.(47) Despite greater than expected amounts of missing data, and although both groups' lung function declined over the 12-months study period, the investigators observed significantly higher FVC (by 0.18 liters) and DlCO (by 0.75mmol/min/kPa) in the group randomized to the NAC-containing regimen. There were also significantly fewer adverse events related to bone marrow toxicity in the group receiving the NAC-containing regimen.
Because the study lacked a true (i.e., inactive) placebo group, caution must be exercised in drawing too many inferences from these results.
Addressed eloquently in an accompanying editorial, one could posit that the combination of prednisone and azathioprine has detrimental effects on lung function in patients with IPF, and NAC, by some mechanism, limits those effects.(48) In any case, because NAC is inexpensive, appears to be well-tolerated, and does not interact substantially with other medications, while recognizing the constraints of the recent study, it seems reasonable to offer NAC to patients with IPF for whatever beneficial effects it may have.
Antibody to connective tissue growth factor. Connective tissue growth factor (CTGF) potently enhances fibroblast proliferation and chemotaxis and fibroblast-induced extra-cellular matrix deposition.(49) In mesenchymal cells, TGF-b is primarily responsible for CTGF induction, and the prevailing theory is that CTGF accounts for many of the pro-fibrotic activities attributed to TGF-b.(49) Expression of CTGF is increased in patients with IPF,(50) and downregulation of CTGF may offer protection from fibrosis as well as clinical improvement.(40) Results from a phase I study presented at the 2004 meeting of the American College of Chest
Physicians showed that a single infusion of either 1mg or 3mg of anti-CTGF per kg body weight was safe, well tolerated, and led to plasma levels of anti-CTGF that remained above predicted minimum effective concentrations (based on animal models) for at least five days.(51) Further studies of anti-CTGF therapy are awaited.
Antibodies to TGF-b. TGF-b comprises an entire family of peptides with similar biologic function; however, only TGF-b1 is consistently found to be up-regulated at sites of lung fibrosis.(49) Within the epithelial-fibroblastic paradigm of IPF pathogenesis, TGF-b appears to play a central and pivotal role. It is a powerful fibroblast chemo-attractant, and it is usually regarded as the most potent stimulator of fibroblast collagen production.(49) It is present in the healthy lung, but increases in message and protein are found in several pathologic states, including IPF.(52) A phase I, multi-centered, open-labeled, single-dose study of a humanized, monoclonal anti-TGF-b1, 2, and 3 antibody for the treatment of IPF is currently underway.
Bosentan. Endothelins are peptides with vasoactive, mitogenic, bronchogenic, and immunomodulatory activity. There are three isoforms of endothelin (ET)-1, -2, and -3. ET-1 is the best characterized and most abundant, and it exerts its effects through two receptors, called ETA and ETB. ET-1 has been shown to induce TGF-b;(53) stimulate fibroblast proliferation, migration, and differentiation into myofibroblasts;(54) stimulate collagen synthesis; and inhibit collagen degradation.(55) In human systems, bronchoalveolar lavage (BAL) and plasma levels of ET-1 are increased in IPF.(56-57) Based on these data, a double-blind, randomized, placebo-controlled, multi-centered trial was undertaken to assess the safety and efficacy of the non-selective ET-1 receptor antagonist (bosentan) in patients with IPF. This study, called Bosentan use in interstitial lung disease, or BUILD-1, is examining six-minute walk distance as a primary outcome. Results should be available in early 2006.
Etanercept. Tumor necrosis factor (TNF)-a is a cytokine, which has been shown to stimulate fibroblast proliferation and collagen gene up-regulation via a TGF-b or platelet-derived growth factor (PDGF) pathway. Interestingly, TNF-a has also been shown to suppress collagen gene expression, thus making it an attractive target in IPF to evaluate further. Niden et al., recently conducted a single-centered, open-labeled, pilot study of the TNF-a blocker, etanercept, in nine IPF patients who had an average ppFVC of 48 and an average ppDlCO of 33.(58) After a mean follow-up of 19 months on twice-weekly etanercept along with prednisone 10mg daily, ppFVC had improved (by >15%) in two, declined (by >15%) in one patient, and remained stable in six patients, while ppDlCO had improved in two, declined in four, and remained stable in two patients. A phase II, 48-week, double-blinded, placebo-controlled trial of the efficacy and safety of etanercept for the treatment of IPF has been completed, and the initial results were presented at the 2005 meeting of the American College of
Chest Physicians. At 48 weeks, lung function had declined from baseline in both groups. Although the primary endpoint was not met, post hoc analyses revealed trends toward significantly lesser declines in absolute- and ppFVC (placebo group decline 5.4% vs. etanercept 2.5%, P=0.10) and absolute and ppDlCO (placebo group decline 4.8% vs. etanercept 2.4%, p=0.16) in the group receiving etanercept. These results will likely fuel further investigation of this drug.
Imatinib. Imatinib mesylate is an oral tyrosine kinase inhibitor. It is a designer molecule developed to treat chronic myelogenous leukemia (CML). In CML, imatinib inhibits the aberrant bcr-abl tyrosine kinase, but is also known to inhibit the receptor tyrosine kinases for platelet-derived growth factor (PDGF).(59) As PDGF has been shown to have significant pro-fibrotic effects, inhibiting its signaling pathway may be anti-fibrotic. A phase II, randomized, double-blinded, placebo-controlled trial of the efficacy and safety of imatinib mesylate for the treatment of IPF is currently enrolling patients. The sample size goal is 100 patients, and the primary combined endpoint is progression of IPF (>10% decline in ppFVC) or death.
Inhaled prostacyclin analog (iloprost). Prostacyclin (prostaglandin I2 or PGI2) is a potent vasorelaxant. In intravenous form (epoprostenol), it has been used extensively and successfully to treat severe PAH. Recently, an inhaled prostacyclin analog (iloprost) was approved by the United States Food and Drug Administration to treat PAH in patients with profoundly symptomatic (New York Heart Association Classes III-IV) disease. In patients with PAH due to lung fibrosis, inhaled prostacyclin is attractive because it limits the systemic vasodilatory/hypotensive effects often seen with intravenous preparations, and, because of its extreme pulmonary selectivity, it may maintain ventilation/perfusion matching to a greater degree than oral or intravenous pulmonary anti-hypertensive preparations.
A phase II, multi-center, double-blinded, placebo-controlled, safety and efficacy trial of iloprost for the treatment of IPF-related PAH is currently underway.
CONCLUSIONSIPF remains a debilitating and deadly disease. Currently, no medical therapies have been proved to prolong survival, stabilize disease, or improve quality of life. However, recent discoveries have dramatically improved our understanding of many facets of this disease. As knowledge accumulates, the list of potential therapeutic targets and agents directed against them grows. Several carefully designed, multi-centered trials evaluating these agents have shown encouraging results, and there are many more trials on the horizon. Hopefully, the momentum created by these recent research efforts will continue to build and generate answers to an increasing number of important questions related to IPF.
REFERENCES 1. American Thoracic Society/ European Respiratory Society International.Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304. Erratum in: Am J Respir Crit Care Med. 2002;166(3):426.
2. Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967-72.
3. Bjoraker JA, Ryu JH, Edwin MK, Meyers J, Tazelaar HD, Schroeder DR, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199-203.
4. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646-64.
5. Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193-6. Comment in: ACP J Club. 2002;136(2):70; Am J Respir Crit Care Med. 2001;164(2):185-6.
6. Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084-90.
7. Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J. 2005;25(1):96-103.
8. Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171(10):1150-7.
9. Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid treatment and its effect on survival. Thorax. 1980;35(8):593-9.
10. Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(5):538-42. Comment in: Am J Respir Crit Care Med. 2004;169(9):1075-6; author reply 1076.
11. Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168(5):531-7. Comment in: Am J Respir Crit Care Med. 2003;168(5):510-1.
12. Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(5):543-8.
13. Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4): 2393-9. Comment in: Chest. 2005;128(4):1897-8.
14. Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360(9337):895-900. Comment in: Lancet. 2002;360(9337):886-7; Lancet. 2003;361(9353):262-3; author reply 263.
15. Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963-7. Summary for patients in: Ann Intern Med. 2005;142(12 Pt 1):123.
16. Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22(5):821-6. Comment in: Eur Respir J. 2004;23(5):792.
17. Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103(6):1808-12.
18. Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136-51.
19. Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3 Suppl):S93-7.
20. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321-32.
21. Bucala R, Spiegel L, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71-81.
22. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438-46.Comment in: J Cliln Invest. 2004;114(3):319-21.
23. Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113(2):243-52. Comment in: J Clin Invest. 2004;113(2):180-2.
24. Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168(3):318-22. Comment in: Am J Respir Crit Care Med. 2003;168(3):267-8.
25. Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1301-15. Comment in: Am J Respir Crit Care Med. 2002;165(6):845-6; author reply 846.
26. Myers JL, Katzenstein AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest. 1988;94(6):1309-11.
27. Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166(2):173-7.
28. Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164(9):1722-7. Comment in: Am J Respir Crit Care Med. 2001;164(9):1553-4.
29. King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164(6):1025-32. Comment in: Am J Respir Crit Care Med. 2002;165(10):1451; author reply 1451.
30. Huaux F, Louahed J, Hudspith B, Meredith C, Delos M, Renauld JC, et al. Role of interleukin-10 in the lung response to silica in mice. Am J Respir Cell Mol Biol. 1998;18(1):51-9.
31. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3): 319-28.
32. Collard HR, Ryu JH, Douglas WW, Schwarz MI, Curran-Everett D, King TE Jr, et al. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest. 2004;125(6):2169-74.
33. Brown KK, Raghu G. Medical treatment for pulmonary fibrosis: current trends, concepts, and prospects. Clin Chest Med. 2004;25(4):759-72, vii.
34. Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP 3rd. Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin Pharmacother. 2004;5(8): 1671-86.
35. Raghu G, Bozic C, Brown K, Lynch D, Center D, Aguayo S, et al. Feasibility of a trial of interferon beta-1A (IFN- Beta-1A) in the treatment of Idiopathic Pulmonary Fibrosis (IPF) [abstract]. Am J Respir Crit Care Med. 2001;163:A707.
36. Billiau A, Heremans H, Vermeire K, Matthys P. Immunomodulatory properties of interferon-gamma. An update. Ann N Y Acad Sci. 1998;856:22-32.
37. Jaffe HA, Gao Z, Mori Y, Li L, Varga J. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp Lung Res. 1999;25(3):199-215.
38. Pfeffer L, Murphy J, Tamm I. Interferon effects on the growth and division of human fibroblasts. Exp Cell Res. 1979;121(1):111-20.
39. Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, et al. Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporine A in lung fibroblasts. FASEB J. 2001;15(3):797-806.
40. Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341(17):1264-9. Erratum in: N Engl J Med. 2000;342(7):524. Comment in: N Engl J Med. 1999;341(17):1302-4; N Engl J Med. 2000;342(13):974-5; N Engl J Med. 2004;350(17):1794-7; author reply 1794-7; N Engl J Med. 2004;350(2):181-3.
41. Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125-33. Comment in: N Engl J Med. 2004;350(17):1794-7; author reply 1794-7; N Engl J Med. 2004;350(2):181-3.
42. Lurton J, Trejo T, Narayanan A, Raghu G. Pirfenidone inhibits the stimulatory effects of profibrotic cytokines on human lung fibroblasts in vitro [abstract].Am J Respir Crit Care Med. 1996;153:A403.
43. Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061-9.
44. Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040-7. Comment in: Am J Respir Crit Care Med. 2005;171(9):939-40; Am J Respir Crit Care Med. 2005;172(9):1228: Am J Respir Crit Care Med. 2005;172(9):1228-9; author reply 1229.
45. Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156(6):1897-901.
46. Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534-54.
47. Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen H, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschaken J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M; IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. New Engl J Med. 2005;353(21):2229-42. Comment in: N Engl J Med. 2005;353(21):2285-7.
48. Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. New Engl J Med. 2005;353(21):2285-7. Comment in: N Engl J Med. 2005;353(21):2229-42.
49. Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res. 2002;3(1): 13-22.
50. Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999;21(6):693-700.
51. Mageto Y, Flaherty K, Brown K, Fong A, Pharm D, Raghu G. Safety and tolerability of human monoclonal antibody FG-3019, anti-connective tissue growth factor, in patients with idiopathic pulmonary fibrosis Chest. 2004;126(4):773S.
52. Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14(2):131-8.
53. McMillen MA, Sumpio BE. Endothelins: polyfunctional cytokines. J Am Coll Surg. 1995;180(5):621-37.
54. Sun G, Stacey MA, Bellini A, Marini M, Mattoli S. Endothelin-1 induces bronchial myofibroblast differentiation. Peptides. 1997;18(9):1449-51.
55. Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27(12):2130-4.
56. Sofia M, Mormile M, Faraone S, Alifano M, Zofra S, Romano L, et al. Increased endothelin-like immunoreactive material on bronchoalveolar lavage fluid from patients with bronchial asthma and patients with interstitial lung disease. Respiration. 1993;60(2):89-95.
57. Uguccioni M, Pulsatelli L, Grigolo B, Facchini A, Fasano L, Cinti C, et al. Endothelin-1 in idiopathic pulmonary fibrosis. J Clin Pathol. 1995;48(4):330-4.
58. Niden A, Koss M, Boylen C, Wilcox A. An open label pilot study to determine the potential efficacy of TNFR: FC (Enbrel®, Etanercept) in the treatment of usual interstitial pneumonitis (UIP)[abstract]. Am J Respir Crit Care Med 2001;163:A42.
59. Novartis pharmaceuticals. Gleevec prescribing information. 2005 [text on the Internet] [cited 2005 Jul 28]. Available from: http://www.ca.novartis.com/downloads/en/products/gleevec_scrip_e.pdf
60. Travis W, Colby T, Koss M, Rosado-de-Christenson M, Muller N, King Jr T. Idiopathic interstitial pneumonia and other diffuse parenchymal lung diseases. In: King D, editor. Atlas of nontumor pathology: non-neoplastic disorders of the lower respiratory tract. Washington, DC: American Registry of Pathology and the Armed Forces Institute of Pathology; 2002. p.49-233.
________________________________________________________________________________________
* Article written at the National Jewish Medical and Research Center, University of Colorado, Denver (CO), USA.
1. Assistant Professor of Medicine, Interstitial Lung Disease Program, National Jewish Medical and Research Center - Denver, Colorado, USA.
2. Associate Professor of Medicine Director, Interstitial Lung Disease Program, National Jewish Medical and Research Center - Denver, Colorado, USA.
Correspondence to: Kevin K. Brown, MD, Director, Interstitial Lung Disease Program, National Jewish Medical and Research Center, 1400 Jackson Street, Denver, Colorado, 80206, USA.
Phone: (303) 398-1355. E-mail: brownk@njc.org
Submitted: 5 January 2006. Accepted: 5 January 2006.
** A versão integral em português deste artigo é disponível no endereço www.jornaldepneumologia.com.br









